Abstract
CD38 is a multifunctional cell surface protein that is frequently overexpressed on malignant plasma cells as well as on immune suppressive cells within the tumor microenvironment and constitutes a validated immunotherapeutic target in the treatment of multiple myeloma (MM). At ONK Therapeutics we are developing a gene edited, cord blood-derived NK (CBNK) cell product targeting CD38 for treatment of patients with relapsed and/or refractory MM.
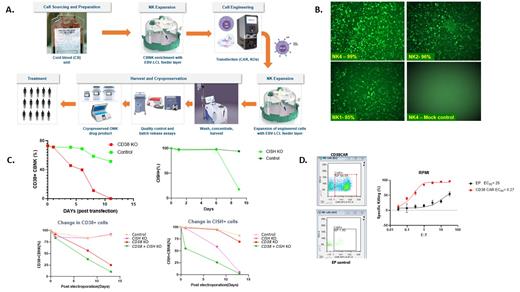
The product will be generated using a workflow shown in Figure 1A. This involves starting with cord blood that is processed for NK expansion using a clinically validated, Epstein Barr Virus-transformed lymphoblastoid cell line (EBV-LCL) feeder layer. The NK cells would undergo genetic engineering that involves gene editing followed by a non-viral chimeric antigen receptor (CAR) introduction process mediated by the TcBuster (TcB) DNA transposon system (Biotechne). This is followed by a second round of expansion on the EBV-LCL feeder layer resulting in a characterized NK cell product that can then be cryopreserved.
In order to develop protocols for optimizing the best transfection efficiencies using the Maxcyte ATx instrument, GFP mRNA (TriLink) was used for transfecting CBNK cells using different electroporation programs. High transfection efficiency was obtained using all programs (Figure 1B.), with the best from program NK4.
Since the product employs an optimized affinity second generation anti CD38 CAR (Stikvoort et al., Hemasphere 2021) which could also target CD38 expressed on neighbouring activated NK cells, it is imperative to knock out (KO) the cell surface expression of CD38 on the CAR-NK cells. To achieve this we carried out CRISPR Cas9 based KO studies of CD38 (Figure 1C. left top), using guide RNAs targeting CD38 (Synthego) in the form of a ribonucleoprotein (RNP) complex with Cas9. CBNK cells were transfected using the Maxcyte ATx instrument and CD38 cell surface expression monitored. As shown in Figure 1C. (left top), complete CD38 KO was achieved 11 days post transfection.
ONK Therapeutics is actively involved in targeting and downregulating the negative regulator of cytokine signalling, cytokine inducible SH2-containing protein (CIS), which is encoded by the CISH gene, as part of their CBNK products. It has been demonstrated that in addition to facilitating greater cytokine signalling, CISH KO also confers greater metabolic capacity to NK cells resulting in their increased persistence (Daher et al., Blood 2021). Therefore, ONK Therapeutics have evaluated CISH KO in CBNK cells (Figure 1C, top right) using the same scheme that was used for the CD38 KO. Guide RNAs in the form of a RNP complex with Cas9 (Synthego) were transfected into CBNK cells and intracellular CIS protein levels monitored over time. Almost complete KO was attained by 9 days post transfection. In order to dial in CISH KO as part of the product, we further carried out a simultaneous KO of CD38 and CISH, in addition to individual KO of CD38 or CISH (Fig 1C, bottom). Simultaneous multiplexing of the CD38 and CISH KOs resulted in efficient double KO (DKO) . The extent of knock down leading to KO in the DKO setting was very similar to that of individual gene KOs.
We then introduced the anti CD38 CAR as part of a transposon that could be transposed by TcB transposase in CBNK cells. After DKO of CD38 and CISH in CBNK cells, the transposon DNA and mRNA for transposase were electroporated. CAR expression was detected 4-5 days post transposition (Figure 1D) with more than 50% of cells expressing the anti CD38 CAR. These CAR expressing CBNK cells were then tested for functionality in a co-culture kill assay against the CD38 positive MM cell line, RPMI8226, which was engineered to express firefly luciferase. In a 4 hour killing assay, robust killing of the RPMI8226 cells was achieved by the CAR-CBNK cells with an EC 50 ten times lower (more potent) than that of mock electroporation control CBNK cells.
To our knowledge this is the first successful expression of an anti CD38 CAR in cord-derived NK cells, and with a double CD38/CISH KO, using non-viral CAR insertion approaches. Current work is focusing on designing and developing a manufacturing-ready workflow for this potential product and further examining the effects of CAR NK cell activity in a DKO setting where both KOs contribute to improved metabolism and potentially NK cell persistence, as well as exploring the added benefit of a DR5 TRAIL variant to enhance cytotoxicity.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal